What is FH
Familial hypercholesterolemia (FH) is a lipoprotein disorder resulting from defects in the hepatic uptake and degradation of LDL particles via the LDL-receptor pathway. It is commonly caused by a loss-of-function mutation in the LDL-receptor gene (LDLR), or by a mutation in the gene encoding apolipoprotein B (APOB) or proprotein convertase subtilisin/kexin type 9 (PCSK9). Rare causative mutations for FH can also lie within several genes such as ARH, APOE, and LIPA. Heterozygous FH is the most common monogenic disorder encountered in clinical practice and affects 1 in 250 individuals.
Affected individuals are characterized by elevations of LDL-Cholesterol > 95th percentile for age and sex, and may show clinical manifestations (xanthomas, xanthelasmas and premature corneal arcus) although these manifestations are seen less frequently with early diagnosis and treatment. Untreated, FH will lead to premature atherosclerotic cardiovascular disease, primarily coronary artery disease, so early detection of affected individuals is a cornerstone of cardiovascular prevention. The most commonly used clinical definitions for FH are the Canadian definition, the Simon Broome Register criteria, the FH Dutch Lipid Clinic Network Criteria, and the MedPed criteria.
In Canada, the simplified Canadian definition for FH is used for the clinical diagnosis of FH, and includes clinical and biochemical features:
- very high LDL-C;
- presence of a known DNA mutation;
- personal history of early cardiovascular disease;
- typical physical findings (stigmata), mainly tendon xanthomas;
- family history of early cardiovascular disease or of marked hyperlipidemia, often requiring treatment.
Diagnostic Criteria:
Diagnostic Criteria - Definition
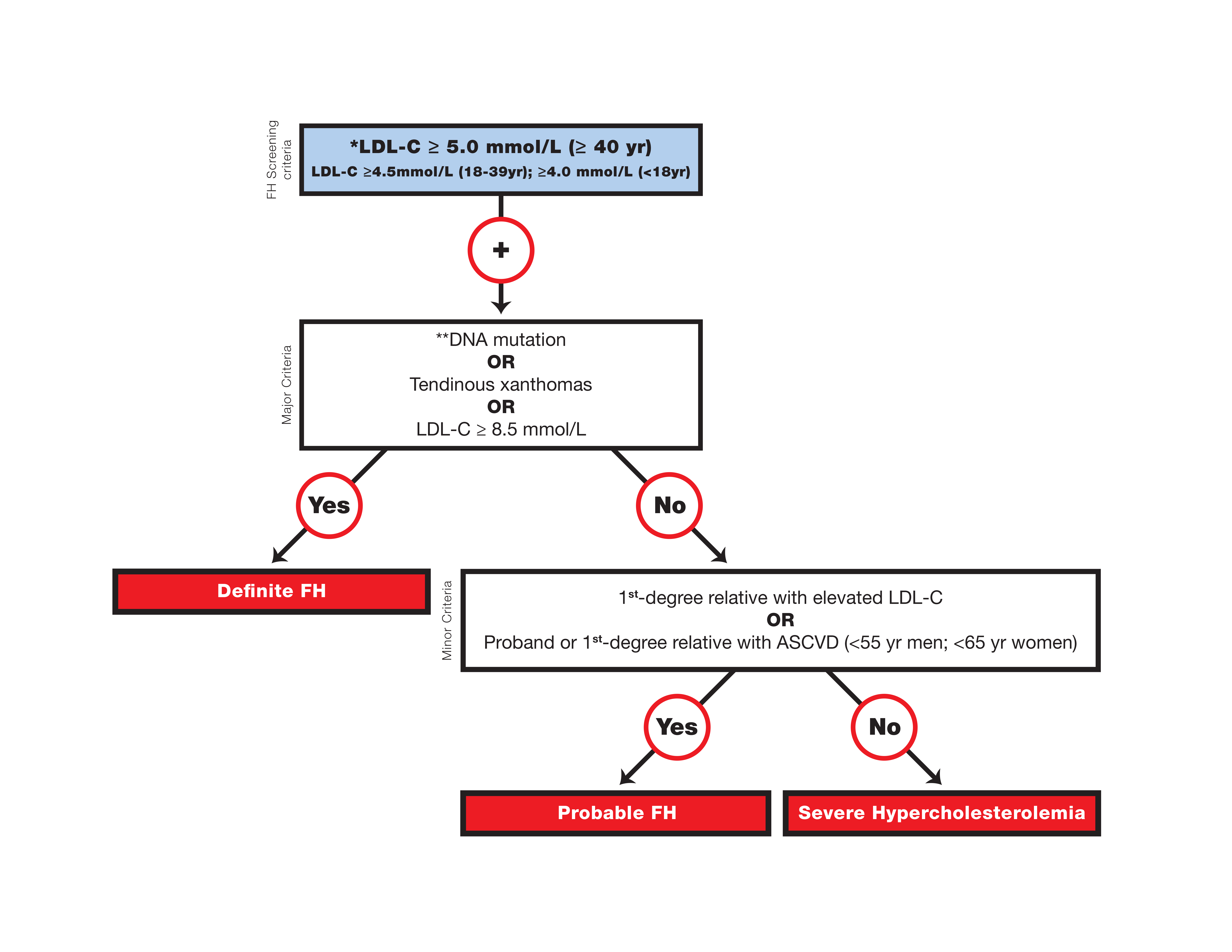
Canadian definition for the clinical diagnosis of familial hypercholesterolemia
* Secondary causes of high LDL-C should be ruled out (severe or untreated hypothyroidism, nephrotic syndrome, hepatic disease [biliary cirrhosis], and medication, especially antiretroviral agents).
** Causal DNA mutation refers to the presence of a known FH- causing variant in the LDLR, APOB, or PCSK9 gene on the basis of presence of the variant in ClinVar, the Human Gene Mutation Database, or Western Database of Lipid Variants databases, in the proband or a first-degree relative. Graphic reproduced from Ruel et al. with permission from Elsevier.
In Canada, it is recommended that genetic testing be offered, when available, to complement a diagnosis of FH and enable cascade screening. The decision to request genetic screening should be made by the treating physician after discussion with the patient (see Genetic testing Section).
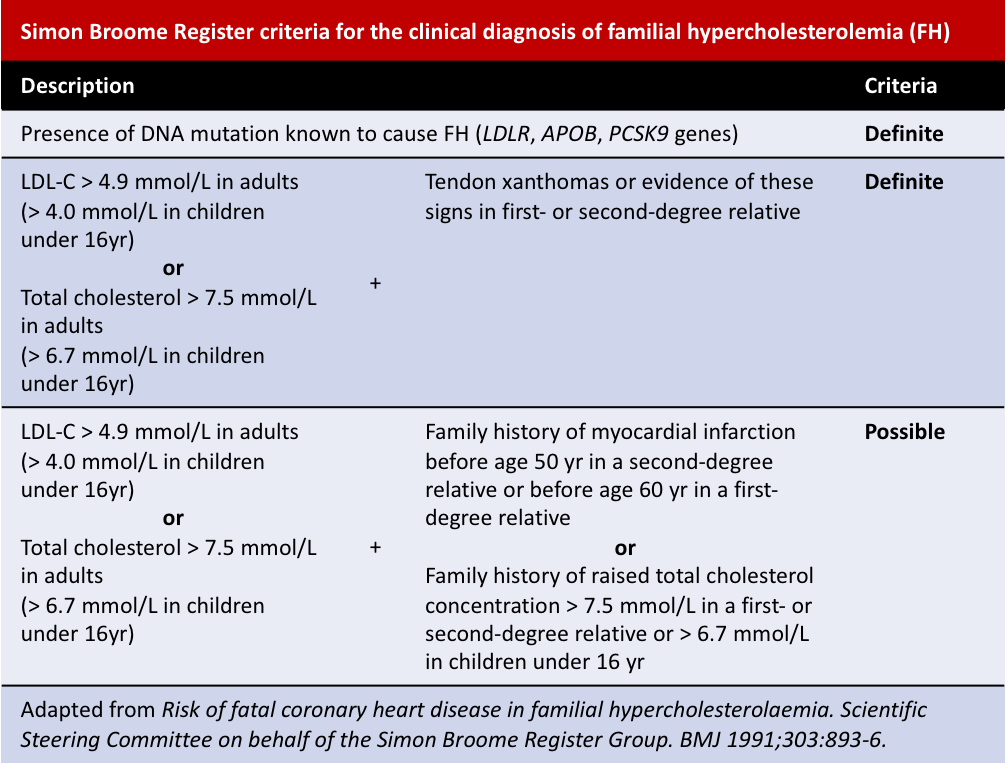
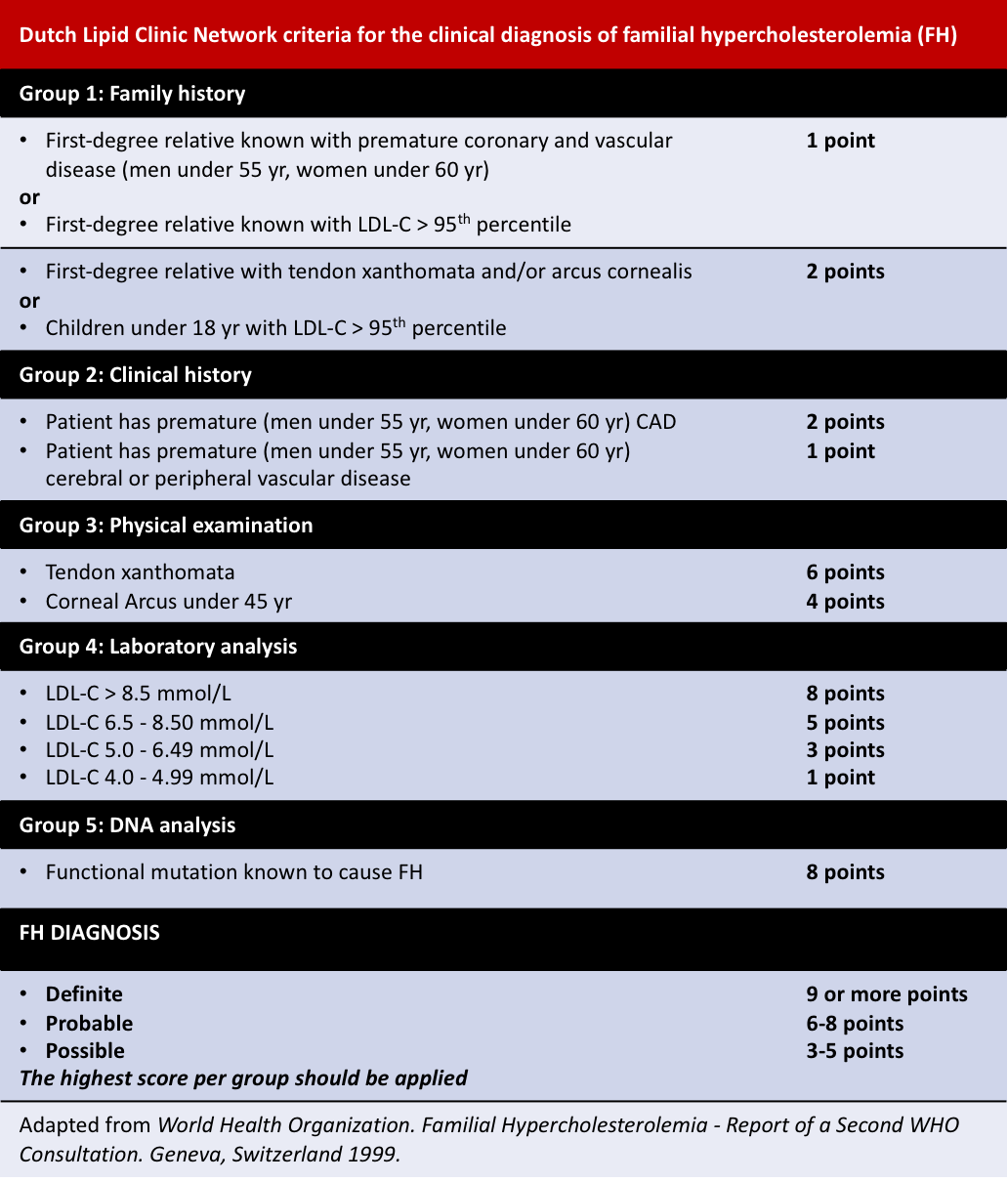
Other diagnosis criteria include the Simon Broome Register and the Dutch Lipid Clinic Network criteria:
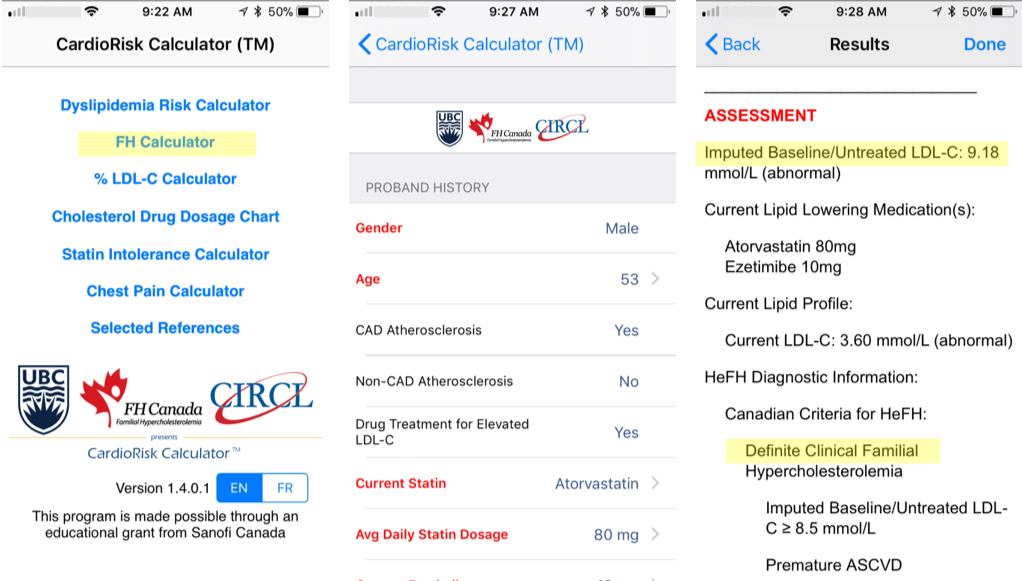
Diagnostic Criteria - CardioRiskTM Calculator

A validated algorithm to impute a baseline value from LDL-C levels while on lipid-lowering therapy is available. The details of the developed algorithm have been published in Clinical Chemistry. This estimated baseline LDL-C for patients with FH help clinicians in making a diagnosis. The imputed baseline LDL-C algorithm is now part of the new “app” for the clinical diagnosis of FH, the FH Calculator, very useful for assessing the degree of severity of FH for new patients and helps facilitate FH diagnosis.
By entering a few characteristics of a patient, the app easily provides a clinical diagnosis of FH based on the Canadian definition as well as the known FH criteria Simon Broome and Dutch Lipid Clinic Network. The tool is freely available to all health care professionals; the computer version generates a report to be saved and added to a patient’s file.
The PC or MAC desktop version are currently available at http://www.circl.ubc.ca/cardiorisk-calculator.html. Click on CardioRisk Calculator™ and install on your computer, or download the “app” directly onto your smartphone or tablet. Also, both Android and iPhone/iOS versions are available through their respective store, in French or in English:
Genetic Testing
Why perform genetic testing for FH?
The benefits of genetic testing for FH are all well summarized in the following video created by Genome British Columbia, which also explained how to interpret and use the genetic tests results: http://www.genomebc.ca/fh-resources
Initiatives are ongoing across Canada to offer a complete genetic assay for the molecular diagnosis of FH. Research genetic testing may be available for patients referred to the Lipid Genetics Clinic, London, Ontario or the BC FH Registry, Vancouver, British Columbia. The Core Molecular Diagnostic Laboratory at the McGill University Health Centre, in Montreal, offers the genetic testing for FH from a clinically-certified laboratory (CLIA #99D1042152). In brief, the algorithm includes 1) testing of the familial variant when known; 2) when no family variant is known: NGS sequencing of the LDLR, APOB and PCSK9 genes (MiSeq); 3) proceed with deletion/duplication analysis of the LDLR gene by MLPA.
Please make sure your patient is eligible for testing by filling out the Eligibility criteria form, filling out the Requisition form, and sending the two forms with two EDTA-lavender blood collection tubes. Do not hesitate to contact the CMDL lab director Dr Jean-Baptiste Rivière for more details:
Treatment Guideline
Several prospective studies have shown that lifetime exposure to high levels of LDL and other atherogenic lipoproteins dramatically increases atherosclerotic cardiovascular disease risk in patients with FH. Individuals with FH-causing variants in the LDLR, APOB, or PCSK9 genes are at a 5- to 22- fold increased risk of atherosclerotic cardiovascular disease compared with normolipidemic individuals, because of lifelong exposure to elevated LDL-C. This risk is further increased by conventional cardiovascular risk factors such as high LDL-C, low HDL-C, age, sex, diabetes, lipoprotein(a) concentrations, body mass index, smoking, and hypertension.
Because the diagnosis of FH using validated clinical criteria and/or genotyping may occur at any age and imparts a high, lifelong risk of ASCVD, we recommend a personalized treatment plan, taking into account at a minimum, age, additional cardiovascular risk factors, psychosocial and socioeconomic factors, and personal as well as family preferences, that should be developed as a shared decision process.
Recommendations for treatment of familial hypercholesterolemia include:
1. Lifestyle modification:
Lifestyle can help modulate the clinical course of FH. As per the Canadian Cardiovascular Society Position Statement on Canadian Cardiovascular Society Position Statement on Familial Hypercholesterolemia: Update 2018, FH subjects and their families would benefit from lifestyle management education, including advice regarding:
- Diet
- Exercise
- Weight control
- Blood pressure control
- Diabetes control
- Smoking cessation; structured smoking cessation programs should be offered to smokers with FH.
More details on the adoption of a healthy lifestyle can be found in the Canadian guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult CCS guidelines for the treatment of dyslipidemia - Canadian Journal of Cardiology 2016.
2. Medication:
LDL-Cholesterol has been shown to be a surrogate for end-points such as cardiovascular death, myocardial infarction, and the need for arterial revascularization. In the absence of randomized controlled trial data of relevance to FH in the current therapeutic era, the general principle should be to attain the lowest level of LDL-C agreed upon between the patient and practitioner; this is with the understanding that randomized controlled trial data from primary and secondary prevention trials suggest that low levels of LDL-C < 1.5 mmol/L are safe and associated with lower residual ASCVD risk.
In patients with FH and ASCVD, the targets should follow the recommendations of the Canadian guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult (LDL-C < 2.0 mmol/L and non-HDL-C < 2.6 mmol/L).
- Statins should be used as the primary line of therapy; statins are the drug of choice for the treatment of familial hypercholesterolemia and in many cases, a 50% reduction of LDL-Cholesterol is achievable with high-dose statins.
- Ezetimibe should be used as second-line agent to achieve unmet LDL-C goals.
- Monoclonal antibody inhibitors of PCSK9 should be considered in adult FH individuals without ASCVD if they have not achieved a 50% reduction in LDL-C from baseline level and reached an LDL-C level of at least < 3.5 mmol/L or lower (as determined by the shared decision process between physician and patient) on maximally tolerated statin therapy with or without ezetimibe.
- Bile acid binding resins (cholestyramine, colestipol or colesevelam) may further lower LDL-C by 10-15%, but complex dosage intervals and side effects may limit their use.
Click here for the “PCSK9: Praluent – Alirocumab Common Drug Review” document
Click here for the “PCSK9: Repatha – Evolocumab Common Drug Review” document
Homozygous FH patients should be referred to a specialized lipid center and undergo complete evaluation for genetic analysis, presence of ASCVD, and aggressive lipid-lowering therapies, including consideration for extracorporeal LDL-C removal, lomitapide, and PCSK9 inhibitors. Novel therapies, including orphan drugs, such as Evinacumab, a fully human antibody directed against angiopoietin-like protein 3 (ANGPTL3), and Gemcabene that lowers LDL-C in a LDLR independent fashion, should be considered in these individuals. Frequent surveillance with cardiovascular imaging and stress testing is necessary to detect and monitor progression of atherosclerosis.
Lifestyle is the cornerstone of preventive strategies in children and all children with a presumptive diagnosis of FH should undergo 6 to 12 months of extensive lifestyle changes including diet, exercise and a tobacco-free environment. If drug treatment is required (if LDL-C remains ≥ 4.9 mmol/L, or ≥ 4.1 mmol/L with a family history of premature ASCVD or other cardiovascular risk factors or risk conditions), statins should be used as first-line therapy with ezetimibe as a second-line agent.
FH Canada Registry for Healthcare Professionals
Click here for the “FH Canada Registry” presentation
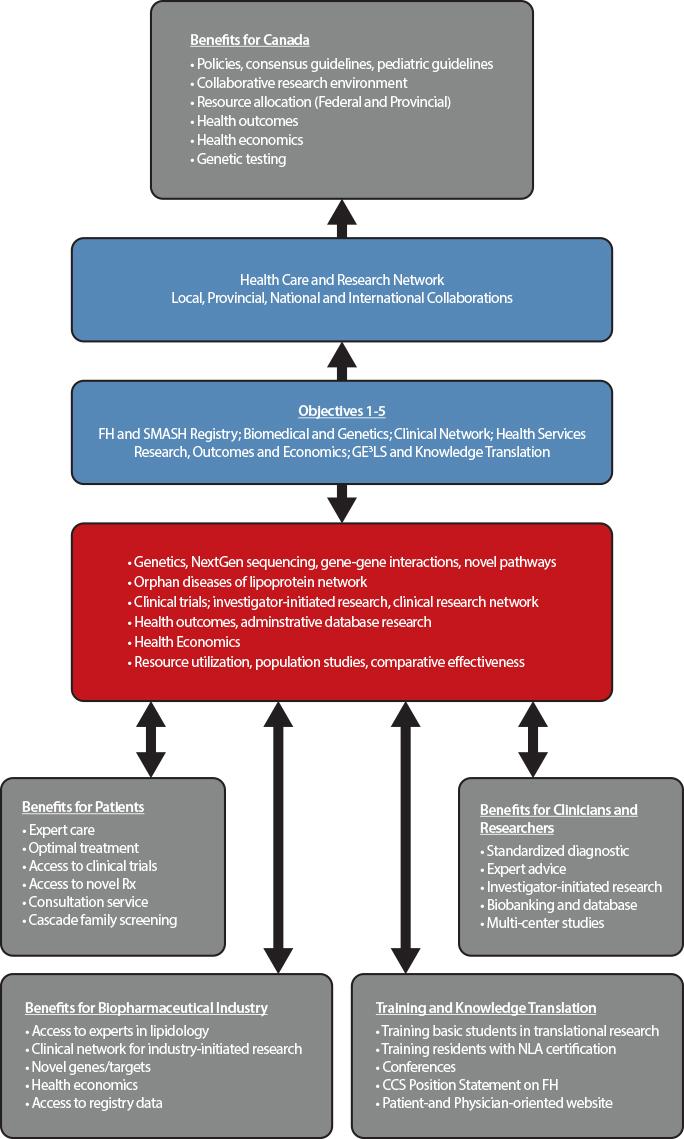
The main goal of the Canadian registry for familial hypercholesterolemia is to improve the identification and initiation of personalized treatment of subjects with FH using the process of cascade screening. This strategy has improved outcomes for subjects with FH in countries in which such registries have been established, including The Netherlands, United Kingdom, and Spain. The FH registry created a network of academic clinics across Canada to promote collaborative clinical research, basic research into the molecular mechanisms of lipoprotein disorders, and crystallized an infrastructure for research on health outcomes and economics, in the broader context of gender, ethical, ethnic, economic, legal and societal aspects of FH.
The national FH Canada registry regroups 19 academic centers across Canada, as well as numerous peripheral sites, in a ‘hub and spoke’ model (Clinicaltrials.gov: NCT02009345). This network comprises over 200 physicians and health care providers who are involved in this initiative. The registry uses a centralized, secure web-based database to allow data entry from the individual sites. The primary inclusion criteria for entry to the Registry is a diagnosis of FH according to either the Canadian definition, the Dutch Lipid Clinic Network (DLCN) or the Simon Broome Registry Criteria.
The James Hogg Research Centre at St‐Paul’s Hospital, UBC, Vancouver is providing the iCAPTURE platform used to capture the data from the FH Canada Registry. Data captured include familial history of elevated cholesterol levels and CVD, the patient’s medical and surgical history, the physical signs of FH, and the patient’s medication profile. It has built-in algorithms to generate an FH diagnosis score using the Canadian definition, Simon-Broome, and Dutch Lipid Clinic Network criteria, as well as to impute a baseline LDL-C value for patients on lipid-lowering medication for which the untreated LDL-C is unknown.
The first results from the FH Canada registry were published in 2018.
Objectives
Our major objective is to identify patients with FH and rare disorders of lipoprotein metabolism, in order to promote access to care and optimize treatment to prevent cardiovascular disease and ultimately improve clinical outcomes.
Our specific objectives are:
- Creation of the Canadian Familial Hypercholesterolemia Registry and the closely related Systems and Molecular Approach to Severe Hyperlipidemias (SMASH) initiative;
- Fostering basic research in genetic dyslipidemia, including discovery of novel genes, novel pathways, and novel targets;
- Leveraging the Registry to drive novel clinical research, including optimizing diagnosis and care in FH patients; and delivery of translational and personalized medicine;
- Using the Registry to promote health services research, including Health Outcomes and Health Economics (for instance, it will permit the evaluation of health services required across the country and the equitable distribution of services where needed, and the examination of the burden of health caused by FH, the requirements for specialized centers (lipid clinics vs. general practitioners) and the need for local expertise and the costs associated with FH in each province);
- Deriving Societal and Education benefits from the Registry, including Genomic, Ethical, Economic, Environmental, Legal and Social (GE3LS) aspects; knowledge translation, education, health promotion and public health policy advice.
What makes this project unique?
- This project aims to be a “projet rassembleur” (unifying project), where clinicians and scientists work together to optimize the care of patients with FH and related lipoprotein orphan diseases;
- The SMASH initiative is unique and will allow patients with rare diseases to have access to expert care and personalized medicine;
- The FH Canada registry helps in the harmonization of treatment recommendations for FH patients across the country;
- A precise diagnosis (Canadian definition for FH and CardioRisk App) allows for personalized medicine and access to new therapeutic modalities and enable early diagnosis and primary prevention treatment by cascade screening of affected relatives;
- This, in turn, will lead to a reduction in CVD and substantial cost savings for Canada;
- It has a strong commitment to knowledge translation to ensure dissemination of new clinical practice guidelines and information on FH for physicians and patients alike.
Why Join
Through the creation of a Canada-wide network of academic clinics, integrating lipid specialists, endocrinologists, cardiologists and general practitioners, the FH Canada registry leads to significant benefits for FH patients, clinicians and researchers, biopharmaceutical industry, and government. The registry is available for clinicians to manage patient care, identify relatives for screening and treatment (cascade screening), provide advice to general practitioners, and to support collaborative studies in biomedical, clinical, health outcomes and health economics research. The data extracted for the provincial portion of the database allows administrative database research that will provide important information to key stakeholders and permit allocation of resources, while also allowing a sound and uniform rationale for the use of novel therapeutic agents and providing expert advice to regulatory agencies. At the Canadian level, the database is useful for clinicians and researchers to determine the burden of disease and the long-term effects of treatment.
Benefits for patients
Patients entered in the FH Canada registry have access to expert care in the field of lipoprotein disorders and cardiovascular disease prevention. In many cases, optimal treatment of severe hypercholesterolemia may be best provided by experts in the field. They have access to on-going clinical trials and to novel therapies. Furthermore, individual patients are offered family screening (cascade screening) to identify affected members who would benefit from early diagnosis and treatment. The identification of new FH cases by cascade screening proves to be both cost-effective and aligns with the aim of providing personalized medicine for patients with severe lipoprotein disorders.
Benefits for clinicians and researchers
For clinicians, this initiative allows a standardized approach to the screening and treatment of FH and, with the SMASH (Systems and Molecular Approach of Severe Hyperlipidemia) initiative, the ability to share information and research material on rare lipoprotein disorders (orphan diseases) between members of the FH Canada registry. The registry provides the local (i.e. clinic or hospital) framework to perform cascade screening (first, second and third-degree relatives), a cost-effective strategy to screen potentially affected subjects. The registry also links clinicians into a network of specialized clinics that favor collaborative investigator-initiated research and participation in multi-center studies. The local biobanking allows the storage of plasma/serum and DNA for future research, after patient’s approval.
For biomedical researchers, the FH Canada registry will help identifying novel genes for FH and other rare lipoprotein disorders that may lead to a better understanding of metabolic pathways and eventually the potential for novel therapeutic targets. In addition, gene-gene and gene-environment interactions research may shed light on mechanisms of disease. The FH Canada registry will allow the clinical-scientists to develop investigator-initiated research, using the pan-Canadian registry and biobanking resources as a platform. For health outcomes and population health researchers, this database will be invaluable to determine the epidemiology, regional disparities, gender effects, ethnic distribution and the effect of therapeutic modalities on outcomes. It will also allow resource allocation according to the prevalence of FH.
Benefits for biopharmaceutical industry
Pharmaceutical and biotechnology industry partners will have access to a group of lipid experts across Canada, with links internationally, and to a harmonized database for subjects with FH and rare lipoprotein disorders. With access to a network of established clinics with a unified database, clinical trials can be rapidly implemented. Access to the registry database will be provided after review of the intended research. This data will be important in determining health economics.
Benefits for government
This initiative will allow the development of clinical practice guidelines, the ability to determine resource allocation according to need, and the identification of rare (orphan disease) lipoprotein disorders requiring specific techniques, such as extracorporeal LDL filtration (apheresis) for homozygous FH. The initiative will allow the framework to set-up collaborative research under the peer-review systems (HSFC, CIHR). Health outcomes and health economic studies will allow resource allocation and quality control. Finally, centralized genetic testing would provide molecular diagnostics in a cost-effective manner when required. This initiative will dovetail with the CIHR C-CHANGE initiative concerning the harmonization of cardiovascular preventive guidelines in Canada. To date, we are the only country with harmonized cardiovascular disease prevention guidelines.
Benefits from knowledge translation and training
Individual academic institutions with a strong biomedical research commitment will offer undergraduate and graduate-level training in the field of lipid and lipoproteins within a country-wide collaborative research environment. Several centers offer post-doctoral fellowships for clinicians and PhDs in lipoprotein metabolism, health outcomes and health economics research. At McGill University, for instance, we have established a Fellowship in Lipidology and Preventive Cardiology. A similar Fellowship is now offered at the University of British Columbia. The training of highly qualified personnel is expected to contribute to the academic and economic engine of the country. A formal training course, aligned with the National Lipid Association (NLA-US), will provide certification on the treatment of lipoprotein disorders. The establishment of clinical practice guidelines for adult and pediatric patients will enable clinicians to hone their clinical practice to the highest standard (Canadian Cardiovascular Society). The creation of patient- and physician-oriented websites will provide the opportunity to disseminate knowledge on FH diagnosis, treatment and research. By using well-established conferences, the aim will be to integrate novel knowledge into the yearly conference programs across Canada and to disseminate this information to practicing physicians and health care professionals.
Additional Resources
Publications and updates
- Canadian Definition for Familial Hypercholesterolemia – Canadian Journal of Cardiology 2018
- 2018 Updated CCS Position Statement on Familial Hypercholesterolemia - Canadian Journal of Cardiology 2018
- FH in Canada: Initial results from the FH Canada Registry – Atherosclerosis 2018
- 2016 CCS guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult - Canadian Journal of Cardiology 2016
- Imputation of baseline LDL-C concentration in patients with FH on statins or ezetimibe – Clinical Chemistry 2018
- FH Canada on ClinicalTrials.gov
- First LDL apheresis in Ontario
Educational resources
- The History of Familial Hypercholesterolemia
- Presentation on the FH Canada Registry
- Familial Hypercholesterolemia for patients
- Other disorders of lipoprotein metabolism
- How to draw genetic family trees
Professional organizations
- Canadian Heart Patient Alliance
- Heart and Stroke Foundation of Canada
- CORD; Canadian Organization for Rare Disorders
- RQMO; Regroupement Québécois des Maladies Orphelines
- US FH Foundation
- The National Lipid Association
- Canadian Cardiovascular Society
Info on drugs
- Alirocumab pregnancy exposure registry
- Evolocumab pregnancy exposure registry overview
- Evolocumab pregnancy exposure registry flyer
- PCSK9: Repatha – Evolocumab Common Drug Review document
- Evolocumab (trade name Repatha), exceptional medication for HeFH in Quebec
- PCSK9: Praluent – Alirocumab Common Drug Review document
Info on the disease
- Hypercholesterolemia, Autosomal Dominant; Online Mendelian Inheritance in Man (OMIM)
- Lysosomal Acid Lipase Deficiency (LALD)
- Learning About Familial Hypercholesterolemia
- Patient’s viewpoint on FH published in Circulation Cardiovascular Quality and Outcomes
- Familial Hypercholesterolemia; National Organization for Rare Disorders
- Learn your lipids
Contact Us
If you think you have FH and would like to register in the FH Canada registry, please find a specialized lipid clinic near you.
If you need additional assistance or have any question about the FH Canada Registry, please contact our National Coordinator.
Contact
Isabelle Ruel, PhD
FH Canada National Coordinator
Research Institute of the McGill University Health Centre
1001 Decarie Boul. E01.3144
Montreal, QC
Canada H4A 3J1
Phone: 514-934-1934 ext 34852
Fax: 514-933-6418
[email protected]





